If you are preparing for board exams or NEET, questions like which one of the following is a weak acid can feel confusing at first. Many students remember acid names but still get stuck in MCQs when multiple familiar options are given together. This is a very common problem in Class 9-12 Chemistry, especially in the chapter Acids, Bases and Salts.
This question is not just about recalling facts. It tests whether a student understands how acids behave in water and how to identify a weak acid based on ionization, not memorisation. That is why the same question pattern appears repeatedly in board exams, school tests, and NEET-level papers.
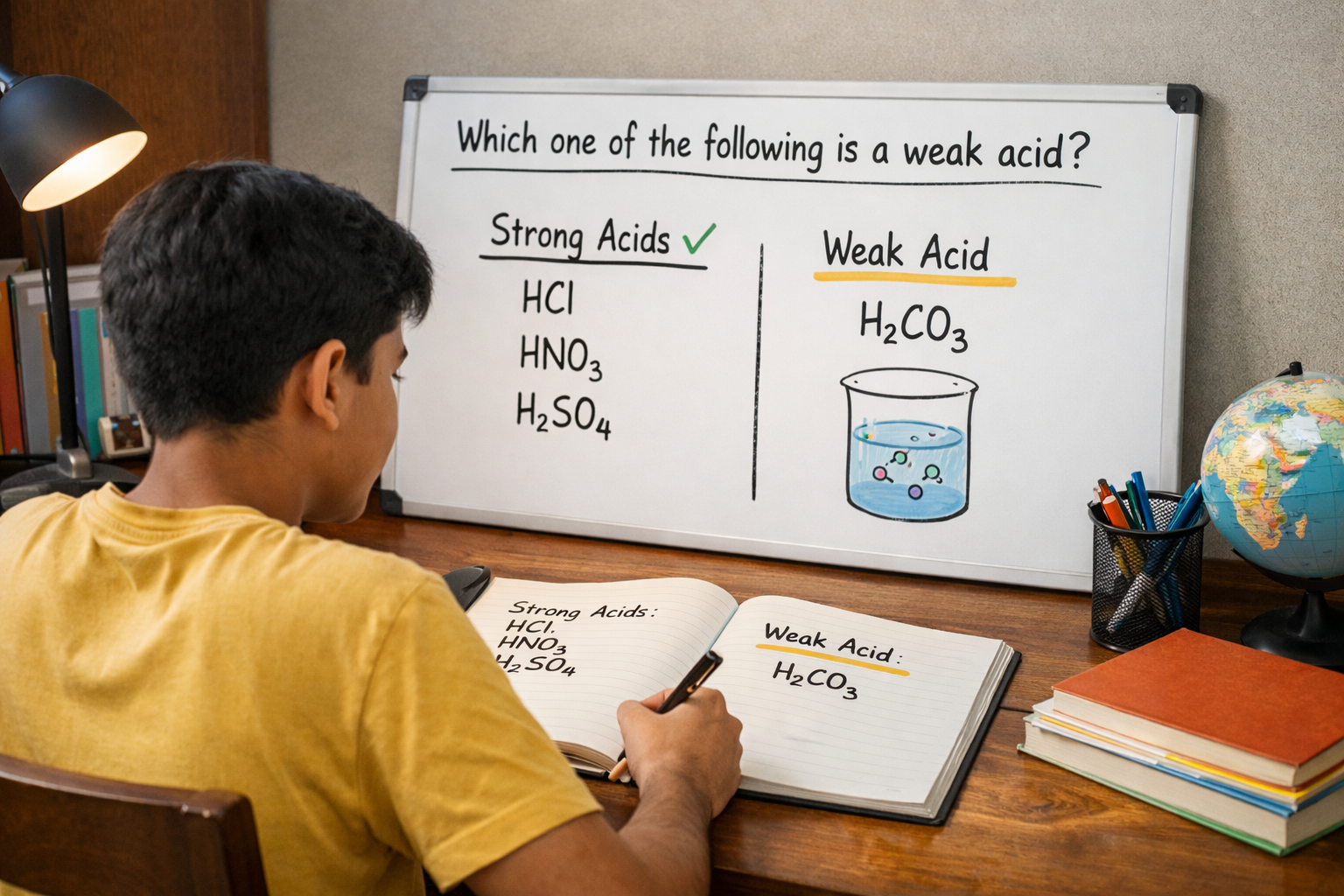
Parents also often notice that their child “knows the chapter” but loses marks in such basic MCQs. The reason is usually unclear concepts, not lack of effort. Once the logic behind weak and strong acids is clear, these questions become easy and predictable.

In this guide, we will clearly explain which one of the following is a weak acid, why it is considered weak, and how students can solve such questions confidently in exams. The explanation is simple, exam-focused, and suitable for both students and parents who want clarity without unnecessary theory.
What Does a Weak Acid Mean in Chemistry?
A weak acid is an acid that does not break completely into ions when it is mixed with water. This means only a small portion of the acid molecules release hydrogen ions (H⁺), while the rest remain unchanged in the solution. Because of this partial reaction, weak acids show milder acidic behaviour compared to strong acids.
In simple terms, the strength of an acid depends on how much it reacts in water. This behaviour is explained using a basic concept called the degree of ionization. It tells us how much of the acid actually separates into ions. For a weak acid, the degree of ionization is low, which is why it does not produce many hydrogen ions.
Students often think that a weak acid is “less important” or “not harmful.” That is not correct. A weak acid can still react and show acidic properties, but the reaction happens slowly and to a limited extent. This is the key difference students need to remember for exams.
For example, when a weak acid is added to water, most of its molecules stay together instead of splitting fully. Only a small number of ions are formed. Because fewer ions are present, the acidic effect is lower compared to strong acids that ionize almost completely.
From an exam point of view, this concept helps students answer MCQs correctly. If an option represents an acid that only partially ionizes in water, it is classified as a weak acid. Understanding this logic is more reliable than memorising long lists of acids.
For parents, this is an important learning point. When a student understands the idea of ionization instead of cramming definitions, their confidence improves and mistakes in basic chemistry questions reduce. A clear understanding of weak acids builds a strong foundation for chapters like acids, bases, salts, and later topics in higher classes.
Strong Acid vs Weak Acid – Key Difference Students Must Know
Ionization in Water (Simple Explanation)
The main difference between strong vs weak acids lies in how they behave when mixed with water. A strong acid breaks almost completely into ions, while a weak acid breaks only partially. This behaviour decides how acidic the solution becomes.
When a strong acid is added to water, most of its molecules separate into hydrogen ions and other ions. Because many ions are formed, the acidic effect is strong and immediate. In exams, such acids are always treated as fully ionising.
A weak acid, on the other hand, releases only a small number of ions in water. Most of its molecules remain in their original form. Due to this partial reaction, a weak acid is often called a weak electrolyte, as it does not conduct electricity well in solution.
For students, remembering this one-point difference can save marks. Instead of memorising names, focus on how much an acid ionises in water. This approach works across board exams and competitive tests.
Why Strong Acids Confuse Students in MCQs
Many students get confused in MCQs because strong acids appear more familiar. Names like hydrochloric acid or nitric acid are commonly taught early, so students tend to choose them without thinking.
Another reason for confusion is that MCQs often mix strong and weak acids together. When students rely only on memory and not on the concept of ionization, mistakes happen easily.
Parents often observe that their child understands theory but loses marks in objective questions. This usually happens when concepts are not applied during exams. MCQs test clarity, not just recall.
To avoid confusion, students should pause and check which acid will ionise fully in water and which will not. If an option forms fewer ions, it falls under the weak acid category.
Once students learn to compare acids using ionization instead of names, strong vs weak acids become easy to identify. This clarity reduces negative marking and builds confidence in chemistry MCQs.
Which One of the Following Is a Weak Acid? (MCQ Explained)
In exams, a very common MCQ is which one of the following is a weak acid HCl, H₂CO₃, HNO₃, H₂SO₄. At first glance, all options look familiar, which is why many students get confused and lose easy marks. The correct answer can be found by using a simple elimination method instead of memorising facts.
Step-by-Step Elimination Logic (Exam Method)
The first step is to identify strong acids that are commonly taught as fully ionising acids. HCl (hydrochloric acid) is a strong acid because it breaks almost completely into ions when dissolved in water. It is widely used in laboratories and is known for its strong acidic nature.
Next, look at HNO₃ (nitric acid). This acid is also classified as a strong acid. In water, it ionises almost fully, releasing a large number of hydrogen ions. In exams, nitric acid is almost never considered weak.
Then comes H₂SO₄ (sulphuric acid). Even though it has two hydrogen atoms, it is still treated as a strong acid at the school and NEET level. Its first ionisation is complete, which is enough to classify it as a strong acid in MCQs.
After eliminating these three, we are left with H₂CO₃ (carbonic acid). This acid does not ionise completely in water. Only a small portion of its molecules release hydrogen ions, while most remain unchanged. Because of this partial ionisation, H₂CO₃ is classified as a weak acid.
So, when asked which one of the following is an example of a weak acid, the correct choice from the given options is H₂CO₃.
For students, this elimination method reduces confusion and saves time during exams. Parents should also encourage children to use logic-based steps like this rather than guessing. This approach improves accuracy and builds confidence in chemistry MCQs.
Common Weak Acids Students Should Remember for Exams
Below are some common weak acids that students should remember for exams. These acids frequently appear in which of the following is a weak acid MCQ type questions in school tests, board exams, and competitive exams. Knowing these examples helps reduce confusion during quick revisions.
- Acetic acid (CH₃COOH):
Acetic acid is a weak acid because it ionises only partially in water. It is commonly taught in the acids bases and salts class 10 chapter and often appears in basic MCQs. - Carbonic acid (H₂CO₃):
Carbonic acid releases very few hydrogen ions in water, which makes it a weak acid. It is formed when carbon dioxide dissolves in water and is a frequent exam example. - Lactic acid (C₃H₆O₃):
Lactic acid is also a weak acid because it does not fully ionise in solution. In chemistry exams, it is mainly used as an example to test concept clarity, not memorisation.
For students, remembering these acids with their behaviour in water is more effective than rote learning. When an MCQ asks you to identify a weak acid, checking whether the acid ionises partially can help you choose the correct answer quickly.
Parents can support their children by ensuring they revise such examples regularly. Clear understanding of common weak acids builds confidence and reduces silly mistakes in chemistry exams.
Why This Question Is Important for Boards & NEET
Questions related to weak and strong acids carry steady importance in board exam chemistry questions, especially in objective sections. These are not surprise questions. They come from core chapters and are used to test whether a student understands basic chemical behaviour.
In board exams, such MCQs are often framed in simple language but require clear thinking. Students who rely only on memorisation tend to make mistakes when options look similar. This is why examiners repeatedly use this pattern to check conceptual understanding.
For competitive exams, this topic is part of NEET chemistry basics. Even though the question looks easy, it can decide marks in high-pressure situations. NEET frequently tests fundamental ideas through direct MCQs rather than lengthy calculations.
From a student’s point of view, mastering this concept improves speed and accuracy. It reduces negative marking and builds confidence in chemistry sections. Parents should also note that strong basics in such topics prevent confusion in higher-level chapters later.
Overall, understanding the logic behind weak acids matters more than memorising names. When students focus on concept clarity, they perform better across both board exams and NEET.
Exam Tip – How to Identify a Weak Acid in 10 Seconds
In exams, do not rush to answer based on familiar names. Pause for a few seconds and think about how each option behaves in water. Ask yourself which acid releases hydrogen ions fully and which one does not.
A quick method is to eliminate acids that are commonly taught as strong. These are usually mineral acids used in labs. What remains is often the weak acid.
Students should practise this method during revision so it becomes automatic in the exam hall. Parents can help by encouraging concept-based revision instead of last-minute memorisation. This habit improves accuracy and reduces stress during chemistry exams.
FAQs – “People Also Ask”
1. Which one of the following is a weak acid in MCQs?
In most MCQs, the weak acid is the one that does not ionise completely in water. Acids like carbonic acid or acetic acid are common correct answers.
2. Is H₂CO₃ a weak acid or a strong acid?
H₂CO₃ (carbonic acid) is a weak acid because it releases only a small number of hydrogen ions when dissolved in water.
3. Why is HCl not considered a weak acid?
HCl is a strong acid because it ionises almost completely in water. Due to this full ionisation, it is never classified as a weak acid in exams.
4. Do weak acids come in board exams?
Yes, questions on weak acids appear regularly in board exams, especially in objective sections from the Acids, Bases and Salts chapter.
5. Are weak acids important for NEET preparation?
Yes, weak acids are part of NEET chemistry basics. Understanding them helps in solving direct MCQs and avoids negative marking.
6. Is acetic acid a weak acid?
Yes, acetic acid is a weak acid because it ionises only partially in water, which reduces its acidic strength.
7. How can students quickly identify a weak acid in exams?
Students should check which acid does not ionise fully in water and eliminate known strong acids first. This method works well in time-based exams.
8. Can weak acids still be harmful?
Yes, weak acids can still be harmful in concentrated form. The term “weak” only refers to partial ionisation, not safety.
9. Why do students get confused between strong and weak acids?
Confusion usually happens when students memorise acid names without understanding how they behave in water. Concept clarity reduces these mistakes.

