Electric dipoles isn’t just about passing your board exams or competitive tests. It’s about grasping one of nature’s most elegant concepts how opposite charges create powerful effects while maintaining perfect balance. Whether you’re preparing for JEE, NEET, or simply want to master Class 12 Physics, this topic is your gateway to understanding everything from molecular bonds to antenna design.
What is an Electric Dipole?
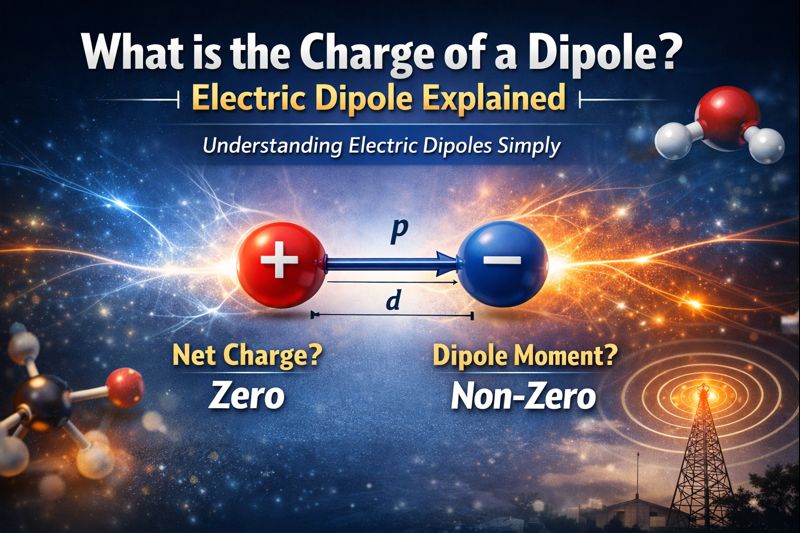
An electric dipole consists of two particles one with a positive charge (+q) and another with an equal negative charge (-q) separated by a small distance.
Think of it like a tiny battery with a positive and negative terminal, but on an atomic scale.
Components:
- Two equal charges: One positive (+q), one negative (-q)
- Fixed separation: Distance between them (represented as 2a or d)
- Opposite nature: Charges are equal in magnitude but opposite in sign
This simple arrangement creates fascinating electrical effects that govern molecular behavior, chemical bonding, and electromagnetic interactions.
What is the Charge of a Dipole?
The net charge of an electric dipole is always zero.
Here’s why: When you add a positive charge (+q) and an equal negative charge (-q), they cancel each other out mathematically.
Net Charge = (+q) + (-q) = 0
Important Distinction:
- Net charge: Zero (charges cancel out)
- Electric dipole moment: Non-zero (creates electric field)
Many students confuse these two concepts. Remember: zero net charge doesn’t mean zero effect. The dipole still creates an electric field and experiences forces in external fields.
Visual Understanding:
| Component | Value |
|---|---|
| Positive charge | +q |
| Negative charge | -q |
| Total charge | +q + (-q) = 0 |
| Dipole moment | p = q × 2a (non-zero) |
Understanding Electric Dipole Moment
Even though the net charge is zero, dipoles have a special property called the electric dipole moment.
Dipole Moment (p) = Charge × Separation Distance
Formula: p = q × 2a
Where:
- p = dipole moment (vector quantity)
- q = magnitude of each charge
- 2a = distance between charges
Direction:
The dipole moment vector points from the negative charge to the positive charge.
Units:
- SI Unit: Coulomb-meter (C·m)
- Common Unit: Debye (D), where 1 D = 3.34 × 10⁻³⁰ C·m
Why It Matters:
The dipole moment tells us the “strength” of the dipole. A larger moment means stronger electrical effects, even though the net charge remains zero.
Why Net Charge Matters
Understanding that the dipole’s net charge is zero helps explain crucial physics concepts:
1. No Response to Uniform Fields
A charged particle accelerates in an electric field. But a dipole with zero net charge experiences no net force in a uniform electric field only a torque that tends to rotate it.
2. Molecular Stability
Water molecules are dipoles. Their zero net charge means they don’t attract every charged particle indiscriminately, yet their dipole moment allows selective bonding.
3. Field Creation
Despite zero net charge, dipoles create their own electric field pattern that decreases faster with distance than single-charge fields.
Classroom Connection:
When your teacher asks about electrostatic force on a dipole, remember: In a uniform field, F = 0 (because net charge is zero), but torque τ = p × E (because dipole moment exists).
Real-Life Examples of Dipoles
1. Water Molecules (H₂O)
Oxygen attracts electrons more than hydrogen, creating partial charges. The molecule has zero net charge but a strong dipole moment (1.85 D).
This is why water dissolves salt and conducts electricity when impure.
2. Carbon Dioxide (CO₂)
Though it has polar bonds, CO₂ is a linear molecule. The two dipole moments cancel out, making it a non-polar molecule overall.
3. Antennas
Radio antennas are essentially oscillating dipoles. They have zero net charge but radiate electromagnetic waves effectively.
4. Proteins in Your Body
Many biological molecules contain dipole regions that determine how proteins fold and function.
Solving Dipole Problems
Example 1: Basic Calculation
Question: An electric dipole consists of charges +2 μC and -2 μC separated by 4 cm. What is the net charge and dipole moment?
Solution:
- Net charge = (+2 μC) + (-2 μC) = 0
- Dipole moment = q × d = 2 × 10⁻⁶ C × 4 × 10⁻² m = 8 × 10⁻⁸ C·m
Example 2: Field Effect
Question: A dipole is placed in a uniform electric field of 10⁴ N/C. What is the net force?
Solution: Since net charge = 0, and the field is uniform: Net force = 0
However, the dipole experiences a torque if not aligned with the field.
Example 3: Comparison
Question: Which has zero net charge: (a) single electron (b) electric dipole (c) charged conductor?
Solution: (b) Electric dipole – because it consists of equal and opposite charges that sum to zero.
Common Mistakes Students Make
Mistake 1: Confusing Net Charge with Dipole Moment
Wrong thinking: “If charge is zero, nothing happens.”
Correct understanding: Net charge is zero, but dipole moment creates real effects.
Mistake 2: Wrong Direction of Dipole Moment
Many students point the vector from positive to negative. Remember: dipole moment points from negative to positive charge.
Mistake 3: Assuming Force is Always Zero
In non-uniform electric fields, dipoles do experience a net force, even though net charge is zero. Only in uniform fields is the force zero.
Mistake 4: Sign Errors
When calculating net charge, forgetting the negative sign on the negative charge leads to wrong answers.
Correct: (+q) + (-q) = 0
Wrong: (+q) + (q) = 2q
Easy Tips to Remember
Memory Trick for Net Charge:
Di-POLE means two POLES positive and negative that CANCEL to zero.
Quick Check:
- One charge? → Net charge = q
- Dipole? → Net charge = 0, but dipole moment ≠ 0
- Uniform field? → Force = 0, but torque may exist
- Non-uniform field? → Both force and torque may exist
Formula Sheet:
| Quantity | Formula | Value for Dipole |
|---|---|---|
| Net charge | Q = q₁ + q₂ | 0 |
| Dipole moment | p = q × 2a | Non-zero |
| Torque in field | τ = p × E sin θ | Depends on angle |
| Force (uniform field) | F = Q × E | 0 |
FAQs about Charge of a Dipole
Q. What is the total charge of an electric dipole?
The total charge of an electric dipole is always zero because it consists of two equal and opposite charges (+q and -q) that cancel each other out completely.
Q. Can a dipole have a non-zero charge?
No, by definition, an electric dipole has equal magnitude opposite charges. If the charges are unequal, it’s not a pure dipole but a charged dipole combination.
Q. Why is dipole moment important if charge is zero?
The dipole moment represents the separation of charge, which creates an electric field and determines how the dipole behaves in external fields, making it crucial for understanding molecular interactions.
Q. Does a dipole experience force in electric field?
In a uniform electric field, a dipole experiences zero net force but may experience torque. In a non-uniform field, it experiences both force and torque.
Q. What is the unit of electric dipole moment?
The SI unit of electric dipole moment is Coulomb-meter (C·m). In chemistry, Debye (D) is commonly used, where 1 Debye equals 3.34 × 10⁻³⁰ C·m.
Q. Is water molecule a dipole?
Yes, water (H₂O) is a polar molecule with a dipole moment of 1.85 Debye due to unequal sharing of electrons between oxygen and hydrogen atoms.
Q. What is the difference between charge and dipole moment?
Net charge measures total charge in a system. Dipole moment measures charge separation and is calculated as charge times distance. A dipole has zero net charge but non-zero dipole moment.
Q. How do you calculate dipole moment?
Dipole moment is calculated by multiplying the magnitude of one charge by the distance between the charges: p = q × d, where direction points from negative to positive charge.
Conclusion
Understanding that an electric dipole has zero net charge is fundamental to mastering electrostatics. The beauty lies in the paradox: zero total charge, yet powerful electromagnetic effects through the dipole moment.
Remember these key takeaways:
- A dipole always has equal and opposite charges (+q and -q)
- The net charge is always zero (they cancel out)
- The dipole moment is non-zero (it depends on charge and separation)
- Zero charge doesn’t mean zero effect dipoles create fields and experience torques
Whether you’re solving numerical problems for your boards, preparing for competitive exams, or simply curious about how molecules work, this concept is your foundation. Practice identifying dipoles in daily life from water molecules to antennas and you’ll never forget this elegant principle of physics.


