What Is a Molecule?
A molecule is the smallest unit of a substance that can exist on its own and still show the properties of that substance. In simple words, when two or more atoms join together, they form a molecule.
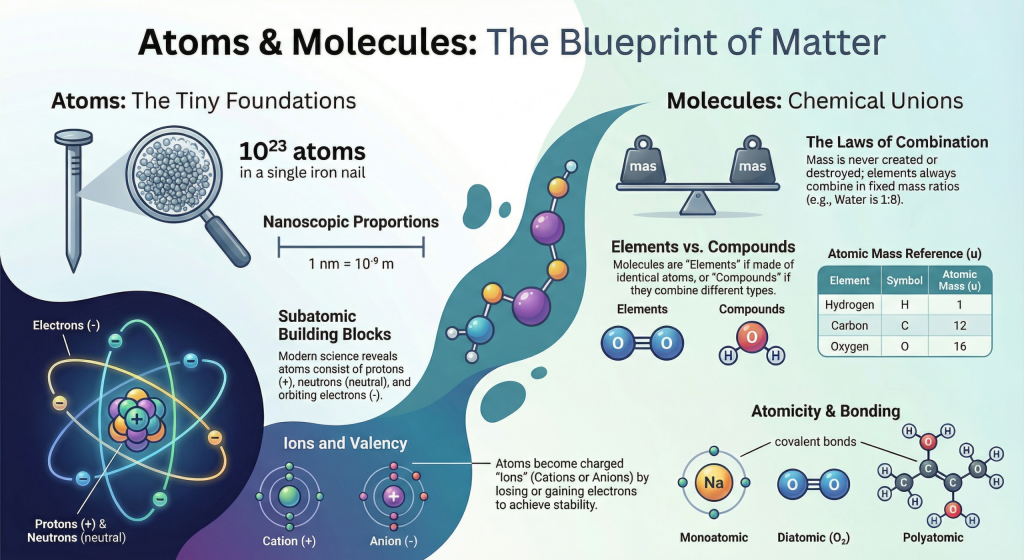
So, if you are wondering what is a molecule, think of it as a small group of atoms that stay bonded and work as one unit.
Simple meaning for students
- An atom is very small
- When atoms combine, they make a molecule
- A molecule can be seen in daily life substances like water and oxygen
Molecule – Simple Definition Table
| Term | Easy Explanation |
| Molecule | A group of atoms joined together |
| Meaning of molecule | Smallest unit of a substance |
| Molecule definition | Unit that keeps the nature of a substance |
Student tip:
This definition is important for school exams, especially from Class 6 to Class 9.
Parent note:
Understanding this concept early helps children avoid confusion later between atom, molecule, and compound.
Definition of Molecule (As Per Chemistry)
In chemistry, a molecule is defined as the smallest part of a substance that can exist independently and still show its chemical nature. This is the commonly accepted definition of molecule used in school-level science.
If students ask what is a molecule definition, the key idea to remember is that a molecule keeps the properties of the substance, even when it is very small.
Key Points to Remember
- A molecule is made when atoms are chemically bonded
- It can exist freely, without breaking into atoms
- It shows the same chemical behaviour as the substance
Chemistry-Based Definition Table
| Term | Explanation |
| Molecule in chemistry | Smallest independent unit of a substance |
| Chemical property | Nature shown by the substance |
| Molecule | Unit that keeps these properties |
Exam note for students:
This wording is suitable for board answers and short-definition questions.
Parent note:
Learning this definition helps children understand later topics like compounds and reactions more easily.
What Is a Molecule Made Up Of?
In science, molecules are made when atoms come together and stay connected. These atoms join in a fixed way to form a stable unit called a molecule.
This joining happens through chemical bonding, which simply means atoms are held together by natural forces. You do not need to remember bonding types at this stage—only understand that atoms do not join randomly.
Simple Breakdown for Students
- Atoms are the building blocks
- Two or more atoms combine
- The combined unit becomes a molecule
Example of a Molecule
| Substance | Atoms Involved |
| Water | Hydrogen + Oxygen |
| Oxygen gas | Oxygen + Oxygen |
Student tip:
Remember this flow: atoms → molecule → substance.
Parent note:
This concept helps children understand how matter is formed in daily life.
Examples of Molecules (Easy to Understand)
Learning with real-life molecule examples makes the concept easier for students. Molecules are present in things we use and breathe every day. Below are some examples of molecules that are commonly taught in school science.
Common Molecule Examples
| Molecule | Atoms Present | Simple Explanation |
| Water (H₂O) | Hydrogen + Oxygen | Forms water used for drinking |
| Oxygen (O₂) | Oxygen + Oxygen | Gas we breathe |
| Carbon Dioxide (CO₂) | Carbon + Oxygen | Gas released while breathing |
Each example of a molecule shows how atoms join in a fixed number to form a stable unit.
Why These Examples Matter
- They are part of daily life
- Easy to remember for exams
- Commonly asked in Class 6–9 questions
Student tip:
Writing these examples helps in short-answer and MCQ-based questions.
Parent note:
Relating science to daily life helps children understand faster and remember longer.
Types of Molecules With Examples
In basic chemistry, types of molecules are explained based on the kind of atoms they contain. This helps students clearly understand how substances are grouped in science exams.
Molecules of Elements
A molecule of an element is made when only one type of atom joins together. Even though there are many atoms, they all belong to the same element.
Examples:
- O₂ – Oxygen molecule
- H₂ – Hydrogen molecule
- N₂ – Nitrogen molecule
These are called molecules of elements because they contain similar atoms only.
| Molecule | Type of Atom |
| O₂ | Oxygen |
| H₂ | Hydrogen |
| N₂ | Nitrogen |
Molecules of Compounds
A molecule of a compound is formed when different types of atoms combine in a fixed ratio.
Examples:
- H₂O – Water
- CO₂ – Carbon dioxide
These are known as molecules of compounds.
| Molecule | Atoms Combined |
| H₂O | Hydrogen + Oxygen |
| CO₂ | Carbon + Oxygen |
Student tip:
Remember this rule for exams: same atoms → element, different atoms → compound.
Parent note:
This classification builds a strong base for higher chemistry topics.
What Is a Molecule for Class 9 Students?
For Class 9 science, students are expected to understand the definition clearly and write it in a proper exam format. When asked what is a molecule class 9, the answer should be short, clear, and accurate.
Definition (Class 9 Level)
Define molecule class 9:
A molecule is the smallest unit of a substance that can exist independently and shows the chemical properties of that substance.
This is the commonly accepted molecule definition class 9 used in NCERT-based answers.
Example for Better Understanding
| Molecule | Explanation |
| H₂O | One molecule of water made from hydrogen and oxygen |
| O₂ | One molecule of oxygen gas |
Exam Tips for Students
- Write the definition first
- Add one correct example
- Keep the answer to the point
Parent note:
Practising this format helps students score well in 1–2 mark questions and builds exam confidence.
Difference Between Atom and Molecule
Students often get confused when learning what is a molecule vs atom. The difference is simple when you focus on size, structure, and role in science.
Atom vs Molecule (Simple Comparison)
| Point | Atom | Molecule |
| Meaning | Smallest unit of an element | Group of atoms joined together |
| Structure | Exists as a single unit | Made of two or more atoms |
| Independent existence | Usually does not exist freely | Can exist independently |
| Example | Hydrogen atom (H) | Water molecule (H₂O) |
How to Remember Easily
- Atom is the basic building block
- Molecule is formed after atoms join
Student tip:
If atoms combine, the result is always a molecule, not another atom.
Parent note:
Clearing this difference early helps children avoid mistakes in exams and higher classes.
Difference Between Molecule and Compound
Many students find it difficult to understand what is a molecule vs compound. The key difference lies in the type of atoms involved, not in size.
Molecule vs Compound (Simple Table)
| Point | Molecule | Compound |
| Meaning | Two or more atoms joined together | Two or more different atoms joined |
| Types of atoms | Same or different | Always different |
| Example | Oxygen (O₂) | Water (H₂O) |
| Scope | Can be element or compound | Always a compound |
Easy Way to Understand
- Oxygen (O₂) is a molecule but not a compound
- Water (H₂O) is both a molecule and a compound
Student tip:
Every compound is a molecule, but not every molecule is a compound.
Parent note:
This clarity helps children answer comparison questions correctly in exams.
Common Student Doubts About Molecules (FAQ)
Q. What is molecule in short answer?
A molecule is the smallest unit of a substance that can exist independently and shows its chemical nature. This idea is used across molecules in science topics.
Q. What is molecular class 9?
In Class 9 chemistry, molecular concepts focus on understanding substances at a very small level, including how atoms join to form molecules.
Q. What is a molecule vs. compound?
A molecule may have same or different atoms, while a compound always has different atoms joined together.
Q. Which best defines a molecule?
In molecule in chemistry, a molecule is defined as a unit that keeps the properties of a substance.
Q. Is every molecule a compound?
No. Oxygen (O₂) is a molecule but not a compound.
Q. Can a molecule exist alone?
Yes. Many molecules can exist independently, like oxygen and nitrogen.
Q. Is oxygen an atom or molecule?
Oxygen gas exists as a molecule (O₂), not as a single atom.
Q. Is mole and molecule the same?
No. Mole is a unit for counting particles, while a molecule is a particle itself.

