The periodic table chart is one of the most important tools in chemistry, but many students feel confused when they first look at it. Rows, columns, symbols, numbers – everything appears together, and without proper explanation, it becomes difficult to understand what to focus on for exams. This confusion often leads students to either blindly memorise the chart or avoid it completely, both of which affect marks.
In reality, the periodic table chart is not meant to be memorised line by line. It is designed to help students understand how the elements in the periodic table are organised, how their properties change, and why certain elements behave in a similar way. For CBSE and ICSE students, this understanding is directly linked to scoring questions in school exams, Olympiads, and even future chemistry concepts in higher classes.

Parents also look at the periodic table as a foundation topic. When this base is clear, students feel more confident while studying reactions, compounds, and properties of elements. Instead of treating it as a complicated chart on the wall, students should learn how to read it correctly and use it as a reference.
This guide explains the periodic table chart in a simple, exam-oriented way, focusing on clarity, understanding, and practical use for students from Classes 6 to 10.
What Is the Periodic Table Chart?
The periodic table chart is a structured way of arranging all known chemical elements so that students can understand their properties more easily. Instead of learning each element separately, this chart shows how elements are connected based on their behaviour and characteristics. This makes chemistry more logical and less confusing for students.
In simple terms, the periodic table of elements is like a map for chemistry. Each box represents one element and gives basic information such as its name, symbol, and position. When students learn how to read this chart, they can quickly find important details during study and revision.
Why Students Learn the Periodic Table Chart
The chart of periodic table of elements helps students see patterns. Elements placed in the same column show similar properties, which is why questions in exams often depend on group trends rather than memorisation. This approach saves time and improves understanding.
From a parent’s point of view, the periodic table chart acts as a foundation topic. When this concept is clear early, students find higher-level chemistry topics easier to handle. It builds confidence and reduces fear of the subject over time.
Modern Periodic Table Chart (CBSE & ICSE Syllabus)
The modern periodic table chart is the version of the table that students study in CBSE and ICSE schools. In this table, elements are arranged mainly according to their atomic number, not their atomic mass. This arrangement helps students understand why elements show certain properties and how these properties change in an orderly way.
The basic idea behind the modern periodic table of elements is simple. When elements are placed in increasing order of atomic number, similar elements naturally fall into the same groups. This makes it easier for students to predict behaviour, compare elements, and answer application-based questions in exams.
For school-level chemistry, students are not expected to memorise all details. The focus is on understanding patterns, such as why elements in one column behave similarly or why properties change across a row. This approach reduces confusion and builds clear thinking from early classes.
Why the Modern Periodic Table Is Used in Schools
Schools follow the modern periodic table chart because it explains chemistry in a logical and scientific way. It avoids the inconsistencies found in older arrangements and gives clearer reasons for similarities among elements.
From a student’s point of view, this structure makes learning easier and less stressful. For parents, it ensures that children are learning concepts that remain valid in higher classes, creating a strong and reliable foundation for future chemistry topics.
Periodic Table Chart With Names and Atomic Numbers
The periodic table chart with names and atomic numbers is the most useful version for students because it gives all basic information in one place. Instead of memorising elements randomly, students can see how elements are arranged and how their position matters in exams.
Below is the complete periodic table with names, atomic numbers, symbols, and element type. Students should use this chart as a study reference, not as something to memorise in one day.
Periodic Table of Elements (Student Reference)
| Atomic No. | Atomic Weight | Symbol | Element Name | Classification |
| 1 | 1.0080 | H | Hydrogen | Non-metal |
| 2 | 4.00260 | He | Helium | Noble gas |
| 3 | 7.0 | Li | Lithium | Alkali metal |
| 4 | 9.012183 | Be | Beryllium | Alkaline earth metal |
| 5 | 10.81 | B | Boron | Metalloid |
| 6 | 12.011 | C | Carbon | Non-metal |
| 7 | 14.007 | N | Nitrogen | Non-metal |
| 8 | 15.999 | O | Oxygen | Non-metal |
| 9 | 18.99840316 | F | Fluorine | Halogen |
| 10 | 20.180 | Ne | Neon | Noble gas |
| 11 | 22.9897693 | Na | Sodium | Alkali metal |
| 12 | 24.305 | Mg | Magnesium | Alkaline earth metal |
| 13 | 26.981538 | Al | Aluminum | Post-transition metal |
| 14 | 28.085 | Si | Silicon | Metalloid |
| 15 | 30.973762 | P | Phosphorus | Non-metal |
| 16 | 32.07 | S | Sulfur | Non-metal |
| 17 | 35.45 | Cl | Chlorine | Halogen |
| 18 | 39.9 | Ar | Argon | Noble gas |
| 19 | 39.0983 | K | Potassium | Alkali metal |
| 20 | 40.08 | Ca | Calcium | Alkaline earth metal |
| 21 | 44.95591 | Sc | Scandium | Transition metal |
| 22 | 47.867 | Ti | Titanium | Transition metal |
| 23 | 50.9415 | V | Vanadium | Transition metal |
| 24 | 51.996 | Cr | Chromium | Transition metal |
| 25 | 54.93804 | Mn | Manganese | Transition metal |
| 26 | 55.84 | Fe | Iron | Transition metal |
| 27 | 58.93319 | Co | Cobalt | Transition metal |
| 28 | 58.693 | Ni | Nickel | Transition metal |
| 29 | 63.55 | Cu | Copper | Transition metal |
| 30 | 65.4 | Zn | Zinc | Transition metal |
| 31 | 69.723 | Ga | Gallium | Post-transition metal |
| 32 | 72.63 | Ge | Germanium | Metalloid |
| 33 | 74.92159 | As | Arsenic | Metalloid |
| 34 | 78.97 | Se | Selenium | Non-metal |
| 35 | 79.90 | Br | Bromine | Halogen |
| 36 | 83.80 | Kr | Krypton | Noble gas |
| 37 | 85.468 | Rb | Rubidium | Alkali metal |
| 38 | 87.62 | Sr | Strontium | Alkaline earth metal |
| 39 | 88.90584 | Y | Yttrium | Transition metal |
| 40 | 91.22 | Zr | Zirconium | Transition metal |
| 41 | 92.90637 | Nb | Niobium | Transition metal |
| 42 | 95.95 | Mo | Molybdenum | Transition metal |
| 43 | 96.90636 | Tc | Technetium | Transition metal |
| 44 | 101.1 | Ru | Ruthenium | Transition metal |
| 45 | 102.9055 | Rh | Rhodium | Transition metal |
| 46 | 106.42 | Pd | Palladium | Transition metal |
| 47 | 107.868 | Ag | Silver | Transition metal |
| 48 | 112.41 | Cd | Cadmium | Transition metal |
| 49 | 114.818 | In | Indium | Post-transition metal |
| 50 | 118.71 | Sn | Tin | Post-transition metal |
| 51 | 121.760 | Sb | Antimony | Metalloid |
| 52 | 127.6 | Te | Tellurium | Metalloid |
| 53 | 126.9045 | I | Iodine | Halogen |
| 54 | 131.29 | Xe | Xenon | Noble gas |
| 55 | 132.905452 | Cs | Cesium | Alkali metal |
| 56 | 137.33 | Ba | Barium | Alkaline earth metal |
| 57 | 138.9055 | La | Lanthanum | Lanthanide |
| 58 | 140.116 | Ce | Cerium | Lanthanide |
| 59 | 140.90766 | Pr | Praseodymium | Lanthanide |
| 60 | 144.24 | Nd | Neodymium | Lanthanide |
| 61 | 144.91276 | Pm | Promethium | Lanthanide |
| 62 | 150.4 | Sm | Samarium | Lanthanide |
| 63 | 151.964 | Eu | Europium | Lanthanide |
| 64 | 157.25 | Gd | Gadolinium | Lanthanide |
| 65 | 158.92535 | Tb | Terbium | Lanthanide |
| 66 | 162.500 | Dy | Dysprosium | Lanthanide |
| 67 | 164.93033 | Ho | Holmium | Lanthanide |
| 68 | 167.26 | Er | Erbium | Lanthanide |
| 69 | 168.93422 | Tm | Thulium | Lanthanide |
| 70 | 173.05 | Yb | Ytterbium | Lanthanide |
| 71 | 174.9667 | Lu | Lutetium | Lanthanide |
| 72 | 178.49 | Hf | Hafnium | Transition metal |
| 73 | 180.9479 | Ta | Tantalum | Transition metal |
| 74 | 183.84 | W | Tungsten | Transition metal |
| 75 | 186.207 | Re | Rhenium | Transition metal |
| 76 | 190.2 | Os | Osmium | Transition metal |
| 77 | 192.22 | Ir | Iridium | Transition metal |
| 78 | 195.08 | Pt | Platinum | Transition metal |
| 79 | 196.96657 | Au | Gold | Transition metal |
| 80 | 200.59 | Hg | Mercury | Transition metal |
| 81 | 204.383 | Tl | Thallium | Post-transition metal |
| 82 | 207 | Pb | Lead | Post-transition metal |
| 83 | 208.98040 | Bi | Bismuth | Post-transition metal |
| 84 | 208.98243 | Po | Polonium | Metalloid |
| 85 | 209.98715 | At | Astatine | Halogen |
| 86 | 222.01758 | Rn | Radon | Noble gas |
| 87 | 223.01973 | Fr | Francium | Alkali metal |
| 88 | 226.02541 | Ra | Radium | Alkaline earth metal |
| 89 | 227.02775 | Ac | Actinium | Actinide |
| 90 | 232.038 | Th | Thorium | Actinide |
| 91 | 231.03588 | Pa | Protactinium | Actinide |
| 92 | 238.0289 | U | Uranium | Actinide |
| 93 | 237.048172 | Np | Neptunium | Actinide |
| 94 | 244.06420 | Pu | Plutonium | Actinide |
| 95 | 243.061380 | Am | Americium | Actinide |
| 96 | 247.07035 | Cm | Curium | Actinide |
| 97 | 247.07031 | Bk | Berkelium | Actinide |
| 98 | 251.07959 | Cf | Californium | Actinide |
| 99 | 252.0830 | Es | Einsteinium | Actinide |
| 100 | 257.09511 | Fm | Fermium | Actinide |
| 101 | 258.09843 | Md | Mendelevium | Actinide |
| 102 | 259.10100 | No | Nobelium | Actinide |
| 103 | 266.120 | Lr | Lawrencium | Actinide |
Tip for students: You are not expected to memorise all elements. Exams test understanding of position, groups, and trends.
Parents can use this chart to help children revise element names and symbols gradually, without pressure.
How to Read Element Names, Symbols & Atomic Numbers
In the periodic table chart with atomic number, every element box gives three key details. The atomic number tells how many protons an element has and decides its position in the table. This number is fixed and very important for exam questions.
The symbol is a short form used in chemical equations. Learning symbols helps students write answers correctly in chemistry papers. The element name connects the symbol to real substances studied in school.
Students should practise reading the chart group by group, not all at once. Parents can encourage slow, regular revision using this chart, which builds confidence and reduces exam fear.
Classification of Elements in the Periodic Table
To make chemistry easier, the elements in the periodic table are grouped based on similar properties. This classification helps students understand behaviour patterns instead of learning each element separately. For Classes 6–10, students only need a clear idea of main groups, not advanced details.
In the chemistry periodic table chart, elements are arranged so that those with similar reactions and uses fall into the same group. This is why questions in school exams often ask about group behaviour rather than individual facts. When students understand these groups, they can answer such questions with more confidence.
From a parent’s view, learning classification early reduces confusion in later chapters like metals and non-metals, acids and bases, and chemical reactions. It builds a strong base and makes revision simpler at home.
Metals, Non-Metals and Metalloids
Most elements in the table are metals. They usually conduct heat and electricity and are used in daily life, such as iron, copper, and aluminium. Students often study their properties and uses in middle school science.
Non-metals behave differently. They may be gases or solids and are important for life processes, like oxygen and carbon. Metalloids fall in between metals and non-metals. They show mixed properties and help students understand why all elements cannot be grouped into just two types.
Alkali Metals, Halogens & Noble Gases (Exam Focus)
Alkali metals are placed in the first group and are known for their high reactivity. Students should remember that these elements react easily and are usually stored carefully. Questions often test this basic idea.
Halogens are reactive non-metals that form salts, while noble gases are mostly unreactive. These groups are frequently asked in exams because their behaviour is easy to compare and remember. Parents can help students revise these groups using simple examples rather than memorising long lists.
Why the Periodic Table Chart Is Important for Exams
The periodic table chart plays a direct role in scoring marks in school exams. Many questions in chemistry are not based on memory alone but on understanding the position of elements. When students know where an element lies in the table, they can answer questions related to properties, reactions, and trends more accurately.
In Classes 6–10, exam papers often include one-mark and short-answer questions that test basic ideas such as group behaviour, metal or non-metal classification, and element symbols. The periodic table chart helps students quickly connect these questions to the correct concept instead of guessing.
From a parent’s point of view, students who regularly refer to the chart make fewer mistakes in chemistry answers. They write more accurate explanations and feel less confused during exams. This steady understanding improves overall performance without extra pressure.
What CBSE & ICSE Students Must Remember
CBSE and ICSE students do not need to memorise the full table. They should remember important groups, common element symbols, and how properties change across rows and columns.
Students must also understand why elements in the same group show similar behaviour. This concept is frequently tested in exams. Parents can support revision by encouraging concept-based learning instead of last-minute rote memorisation.
Picture of the Periodic Table
A picture of the periodic table is meant to support learning, not replace understanding. After students know what the table shows and how elements are arranged, a visual helps them connect names, symbols, and positions more clearly. This makes revision faster and reduces confusion during study.
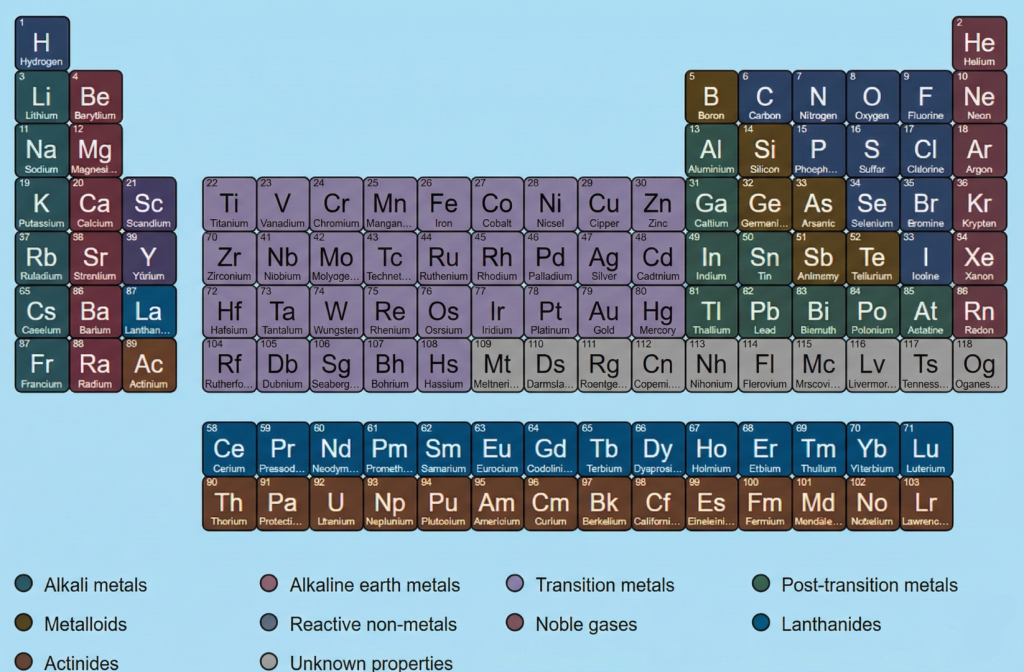
Students should use the image as a reference chart while reading chapters related to elements, metals and non-metals, or chemical reactions. Looking at the table again and again helps students become familiar with element positions without memorising forcefully.
For parents, this visual works as a simple guide to support revision at home. Even without deep chemistry knowledge, they can help children locate elements and revise basic groups.
The picture below shows the complete periodic table in a clear format. Students are advised to refer to it regularly while studying, not just before exams.
Common Mistakes Students Make While Using the Periodic Table
One common mistake students make is trying to memorise the entire periodic table at once. This creates pressure and confusion, especially in lower classes. The table is meant to be understood step by step, not learned like a long list.
Another mistake is ignoring the position of elements and focusing only on names or symbols. Many exam questions are based on where an element is placed, not just what it is called. Missing this connection often leads to incorrect answers.
Some students also mix up metals and non-metals because they do not revise the basic groups properly. This usually happens when revision is rushed. Parents can help by encouraging slow and regular practice using the table as a reference.
Lastly, students often look at the periodic table only before exams. Regular use during study builds familiarity and confidence, which reduces mistakes during the actual paper.
FAQs: Periodic Table Chart
Q. What are the elements from 1 to 118 in the periodic table?
The periodic table has 118 known elements, starting from Hydrogen (1) to Oganesson (118). School exams usually focus only on common elements, not the full list.
Q. Do students need to memorise all 118 elements?
No. CBSE and ICSE exams do not require memorising all elements. Students should understand groups, symbols of common elements, and basic trends.
Q. What element has the electronic configuration 1s² 2s² 2p⁶ 3s² 3p⁴?
This electronic configuration belongs to Sulfur (S). Learning how to link configurations with atomic numbers helps in such questions.
Q. 1s² 2s² 2p⁶ 3s² 3p⁴ कौन सा तत्व है?
यह Sulfur (S) तत्व है। इसका atomic number 16 होता है, जो periodic table से आसानी से पता लगाया जा सकता है।
Q. What is the 2, 8, 8, 18, 18 rule in chemistry?
This rule explains the maximum number of electrons that can be present in different shells. It helps students understand electronic configuration at a basic level.
Q. 6. 2, 8, 8, 18, 18 नियम क्या है?
यह नियम electron shells में electrons की संख्या समझाने के लिए होता है। Exam में इसका concept पूछा जाता है, पूरी theory नहीं।
Q. How is the periodic table useful in exams?
Many questions are based on element position, group behaviour, and properties. The periodic table helps students answer such questions logically.
Q. What information does one box of the periodic table show?
Each box shows the element’s atomic number, symbol, name, and sometimes atomic weight. This information is often directly used in exam questions.
Q. Is the periodic table same for CBSE and ICSE students?
Yes. Both boards follow the modern periodic table. Only the depth of questions may differ based on class level.


