The Finkelstein reaction is an important organic chemistry reaction taught in Class 12 Chemistry, especially in the chapter Haloalkanes and Haloarenes. It explains how one halogen in an alkyl halide can be replaced by another. This reaction is frequently asked in board exams, JEE, and NEET, mainly to test concept clarity and reaction mechanism understanding.
What Is Finkelstein Reaction?
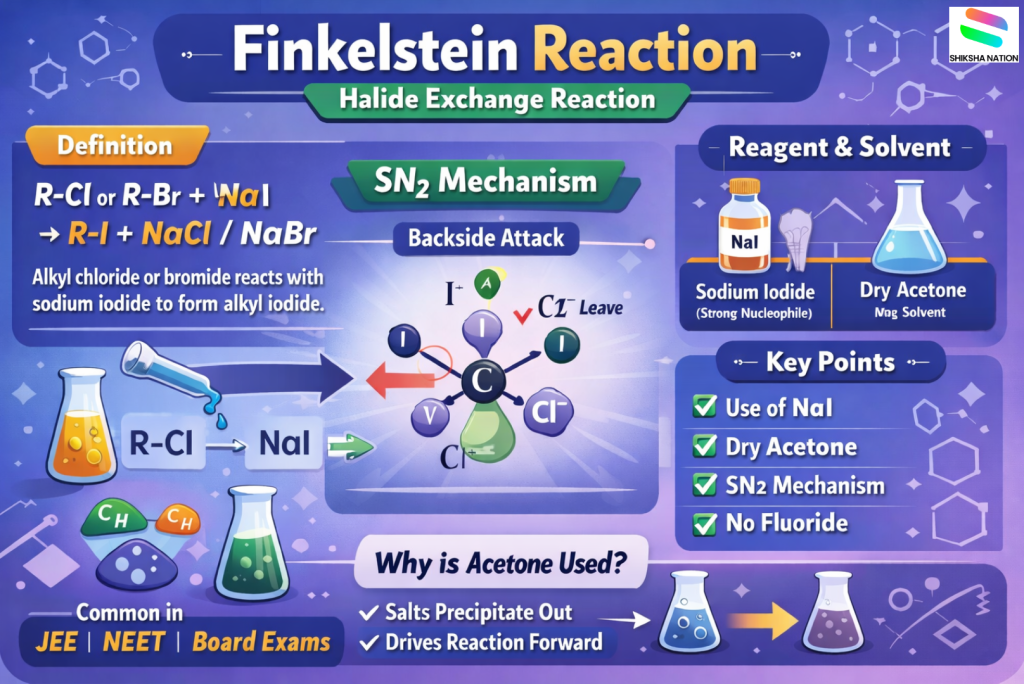
The question what is Finkelstein reaction is commonly asked by Class 12 students while studying reactions of haloalkanes. This reaction explains a simple halogen exchange process and helps students understand how nucleophilic substitution works. Knowing this reaction is important for writing correct answers in board exams and for solving application-based questions in competitive exams.
Finkelstein Reaction Definition
The Finkelstein reaction definition states that it is a chemical reaction in which an alkyl chloride or alkyl bromide is converted into an alkyl iodide by treating it with sodium iodide in a suitable solvent.
Exam-ready answer:
Finkelstein reaction is the conversion of alkyl chlorides or bromides into alkyl iodides using sodium iodide.
Finkelstein Reaction Is Used to Prepare Which Compounds?
The Finkelstein reaction is used to prepare alkyl iodides from alkyl chlorides or alkyl bromides. Iodide ion is a strong nucleophile, so it easily replaces chlorine or bromine.
The reaction moves forward because sodium chloride or sodium bromide formed during the process separates out as a solid. This precipitation helps the reaction complete smoothly, which is why this reaction is reliable and often discussed in exams.
Reagent and Medium Used in Finkelstein Reaction
Understanding the reagent and medium of a reaction helps students answer “why” questions in exams, not just “what.” In the Finkelstein reaction, the correct choice of chemical and solvent decides whether the reaction will occur or not. This topic is especially important for Class 12 students preparing for boards and entrance tests.
Finkelstein Reaction Reagent
The main Finkelstein reaction reagent is sodium iodide (NaI). It provides iodide ions, which take part directly in the reaction.
Iodide ion is a strong nucleophile. Due to its larger size, it can easily attack the carbon atom and replace chlorine or bromine. This is why sodium iodide is preferred over other halide salts in this reaction.
In Which Medium Does Finkelstein Reaction Take Place?
Students often ask in which medium does Finkelstein reaction take place. The reaction is carried out in dry acetone, which acts as the solvent.
Sodium iodide dissolves well in acetone, but sodium chloride and sodium bromide do not. As a result, these salts separate out as solids. This difference in solubility helps the reaction move forward and complete efficiently.
Why Is Dry Acetone Used in Finkelstein Reaction?
Many Class 12 students find this question confusing, yet it is one of the most common board and entrance exam questions. Understanding why acetone is used in Finkelstein reaction helps students give logical answers instead of memorising lines. Parents can also use this explanation to guide students during revision.
The solvent used in a reaction is not chosen randomly. In this case, dry acetone plays a key role in making the reaction possible and effective.
Role of Dry Acetone in Finkelstein Reaction
The role of dry acetone in Finkelstein reaction is mainly related to solubility. Sodium iodide dissolves easily in acetone, so iodide ions are freely available to take part in the reaction.
When the reaction starts, sodium chloride or sodium bromide is formed. These salts are not soluble in acetone, so they separate out as solid particles. This is an important point students should remember for exams.
Because these solids are removed from the solution, the reaction keeps moving in the forward direction. This shift helps in the complete formation of alkyl iodide.
Dry acetone is used instead of moist acetone because water can disturb the reaction process. That is why dry acetone is used in Finkelstein reaction, and this explanation often fetches full marks in “reason-based” questions.
Mechanism of Finkelstein Reaction
The Finkelstein reaction mechanism is important for students preparing for JEE and NEET, as questions often test the type of substitution involved. Instead of memorising steps, students should focus on how and why the reaction happens. This approach helps in solving tricky multiple-choice questions confidently.
Is Finkelstein Reaction SN1 or SN2?
A common exam question is whether Finkelstein reaction is SN1 or SN2. The correct answer is SN2 mechanism.
In this reaction, the iodide ion attacks the carbon atom from the opposite side of the leaving group. This is known as backside attack. Because of this direct attack, the reaction occurs in one step without forming any intermediate.
Primary alkyl halides are preferred because there is less crowding around the carbon atom. This makes it easier for the iodide ion to approach and replace the leaving group.
Key Features of the Mechanism
During the reaction, a short-lived transition state is formed where both the incoming iodide ion and the leaving halogen are partially attached to the carbon.
The strength of the leaving group also matters. Chloride and bromide ions leave more easily in the given conditions, allowing the reaction to proceed smoothly. Understanding these points helps students answer mechanism-based questions accurately in exams.
Finkelstein Reaction Example
Learning a reaction becomes much easier when students see how it works in an actual chemical change. A clear Finkelstein reaction example helps Class 12 students understand the concept and write correct answers in exams. Parents can also use such examples to support practice during revision time.
Explain Finkelstein Reaction With an Example
To explain Finkelstein reaction with an example, consider an alkyl chloride reacting with sodium iodide in dry acetone.
When ethyl chloride is treated with sodium iodide, the iodide ion replaces the chlorine atom. As a result, ethyl iodide is formed.
Chemical equation:
Ethyl chloride + Sodium iodide → Ethyl iodide + Sodium chloride
The sodium chloride formed during the reaction does not dissolve in acetone and separates out as a solid. This helps the reaction move forward and complete properly. Such examples are commonly asked in board exams, especially in short-answer and reasoning-based questions.
Where Is Finkelstein Reaction Taught in Class 12?
Many students and parents want to know exactly where this reaction fits in the Class 12 syllabus. This helps in better planning of study time and focused revision before exams. Knowing the chapter also helps parents guide students using the correct NCERT book and reference material.
Finkelstein Reaction Class 12 – Chapter Reference
If you are searching finkelstein reaction class 12 which chapter, it is taught in the chapter “Haloalkanes and Haloarenes” of NCERT Class 12 Chemistry.
This chapter explains different reactions of alkyl halides, including substitution reactions. Finkelstein reaction is discussed as an example of halogen exchange and is important for both theory questions and mechanism-based problems in exams.
Exam-Oriented Notes on Finkelstein Reaction
This section is useful for students during last-minute revision and for parents helping with exam preparation. Many board exam questions directly ask to write a short note on Finkelstein reaction, so clear and structured points are important.
Write a Short Note on Finkelstein Reaction
- Define Finkelstein reaction: It is a chemical reaction in which an alkyl chloride or alkyl bromide is converted into an alkyl iodide using sodium iodide.
- The reagent used is sodium iodide (NaI), which provides iodide ions for the reaction.
- The reaction takes place in dry acetone, which acts as a suitable solvent.
- It follows an SN2 type mechanism, involving a single-step nucleophilic substitution.
- The reaction moves forward due to the separation of sodium chloride or sodium bromide as a solid.
These points are enough to score full marks in short-answer questions.
FAQ’s on Finkelstein Reaction
This section answers common doubts asked by students during revision and by parents while helping with homework. These questions often appear in exams or oral tests, so clear understanding is important.
Q. What is Finkelstein reaction in simple words?
In simple terms, Finkelstein reaction is a process where one halogen in an organic compound is replaced by another. To explain Finkelstein reaction simply, it changes an alkyl chloride or bromide into an alkyl iodide using a suitable chemical and solvent. The reaction is easy to remember and commonly tested in Class 12 exams.
Q. Why acetone is preferred over water?
Students often ask why acetone is used in Finkelstein reaction instead of water. Acetone helps the reaction move forward because some salts dissolve in it while others do not. Water would dissolve all salts and stop the reaction from completing properly, which is why it is avoided.
Q. Can secondary alkyl halides undergo this reaction?
Secondary alkyl halides can take part in this reaction, but the process is slow. This happens due to crowding around the carbon atom, which makes it difficult for the iodide ion to attack. Primary alkyl halides react more easily and are preferred in exams.
Q. Why fluoride is not prepared by Finkelstein reaction?
Fluoride ions are very small and strongly attached to sodium. Because of this strong attraction, they do not replace other halogens easily. So alkyl fluorides are not prepared using this reaction.


