Organic chemistry becomes easy when we understand reactions step by step. One important topic in Class 11 and Class 12 Chemistry is the preparation of ethyl bromide. In this article, we will clearly explain how ethyl bromide is prepared from ethyl alcohol (ethanol), ethane, and ethene, along with reaction equations, mechanisms, and important exam points.
We will use simple language so that students can easily understand the concept.

What is Ethyl Bromide?
Ethyl bromide is an organic compound. Its IUPAC name is bromoethane.
Basic Information:
- Chemical Formula: C₂H₅Br
- IUPAC Name: Bromoethane
- Class: Haloalkane / Alkyl halide
- Type: Primary alkyl halide
Ethyl bromide is formed when one hydrogen atom of ethane is replaced by a bromine atom. It is a colorless liquid and is used in organic synthesis and laboratory reactions.
Preparation of Ethyl Bromide
Ethyl bromide (C₂H₅Br) can be prepared in three main ways:
- From Ethyl Alcohol (Ethanol)
- From Ethane
- From Ethene
Let us understand each method in detail.
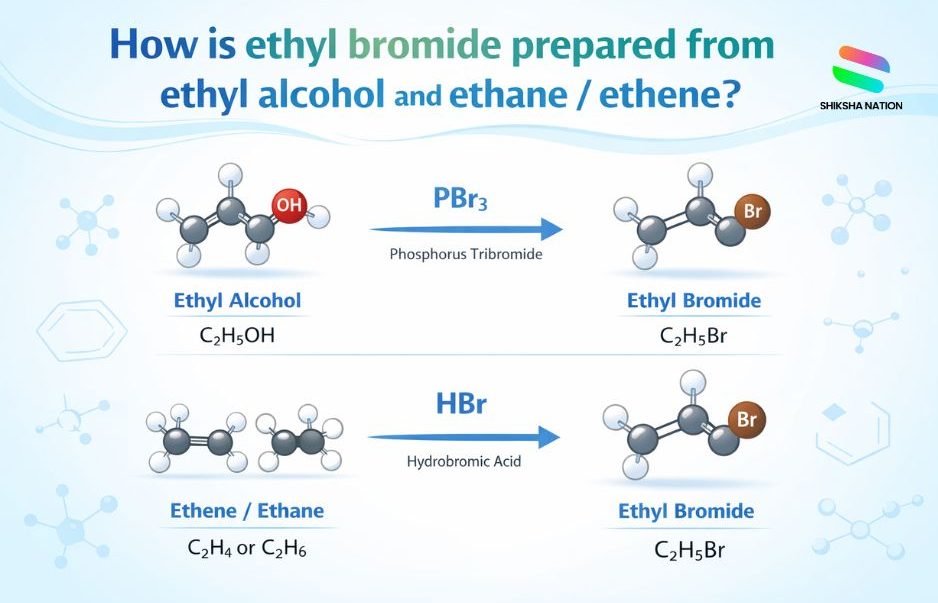
1. Preparation of Ethyl Bromide from Ethyl Alcohol (Ethanol)
This is the most common laboratory method.
Reaction Involved:
When ethanol reacts with hydrobromic acid (HBr), ethyl bromide is formed.
Chemical Equation:
C₂H₅OH + HBr → C₂H₅Br + H₂O
Other Reagents Used:
- Phosphorus tribromide (PBr₃)
- Red phosphorus + Bromine
Type of Reaction
This reaction is a nucleophilic substitution reaction (SN2 reaction).
In this reaction:
- The OH group of alcohol is replaced by Br.
- Water is formed as a by-product.
Mechanism
- The –OH group of ethanol is not a good leaving group.
- HBr converts –OH into water (H₂O).
- Bromide ion (Br⁻) attacks the carbon.
- Water leaves.
- Ethyl bromide is formed.
Since ethanol is a primary alcohol, it follows the SN2 mechanism.
Important Exam Points
- Best method for laboratory preparation.
- Gives better yield.
- Commonly asked in board exams.
- Example of substitution reaction of alcohol.
2. Preparation of Ethyl Bromide from Ethane
Ethyl bromide can also be prepared from ethane by bromination.
Reaction Involved:
C₂H₆ + Br₂ → C₂H₅Br + HBr
This reaction takes place in the presence of UV light or sunlight.
Type of Reaction
This is a free radical substitution reaction.
It is also called:
- Halogenation of alkane
- Photochemical reaction
Mechanism of Free Radical Reaction
The reaction occurs in three steps:
1. Initiation
Br₂ breaks into two bromine radicals under UV light.
Br₂ → 2Br•
2. Propagation
Bromine radical reacts with ethane.
Br• + C₂H₆ → C₂H₅• + HBr
C₂H₅• + Br₂ → C₂H₅Br + Br•
3. Termination
Two radicals combine to stop the reaction.
Important Points
- Requires UV light.
- Not very selective.
- Can form side products.
- Used mainly for industrial purposes.
3. Preparation of Ethyl Bromide from Ethene
Ethyl bromide can be prepared from ethene by adding hydrobromic acid.
Reaction Involved:
C₂H₄ + HBr → C₂H₅Br
Type of Reaction
This is an electrophilic addition reaction.
It is also called:
- Hydrohalogenation of alkene
- Addition reaction of ethene
Mechanism
- Ethene contains a double bond.
- The double bond breaks.
- H⁺ attaches first.
- Br⁻ attaches next.
- Ethyl bromide is formed.
Role of Markovnikov’s Rule
In unsymmetrical alkenes, hydrogen attaches to the carbon with more hydrogen atoms. But ethene is symmetrical, so only one product is formed.
Comparison of All Three Methods
| Starting Compound |
Type of Reaction |
Mechanism |
Condition Required |
Best Use |
| Ethanol |
Substitution |
SN2 |
HBr / PBr₃ |
Laboratory |
| Ethane |
Substitution |
Free Radical |
UV Light |
Industrial |
| Ethene |
Addition |
Electrophilic |
Normal temperature |
Simple preparation |
Difference Between Substitution and Addition Reaction
Substitution Reaction
- One atom replaces another.
- Example: Ethanol → Ethyl bromide
- Occurs in alkanes and alcohols.
Addition Reaction
- Atoms are added across double bond.
- Example: Ethene + HBr
- Occurs in alkenes.
Mechanism Summary (For Quick Revision)
SN2 Reaction
- One-step reaction.
- Backside attack.
- No intermediate.
- Seen in primary alcohol.
Free Radical Reaction
- Involves radicals.
- Needs UV light.
- Occurs in alkanes.
Electrophilic Addition
- Double bond breaks.
- Carbocation intermediate formed.
- Occurs in alkenes.
Which Method is Best?
- For laboratory preparation → Ethanol method
- For industrial production → Ethane method
- For simple textbook explanation → Ethene method
Important Board Exam Questions
- How is ethyl bromide prepared from ethanol?
- Write the mechanism of bromination of ethane.
- Explain addition of HBr to ethene.
- What type of reaction converts alcohol to alkyl halide?
- Why does ethanol follow SN2 mechanism?
Uses of Ethyl Bromide
- Used in organic synthesis.
- Used to prepare Grignard reagent.
- Used in laboratory experiments.
- Intermediate in chemical industry.
Conclusion
Ethyl bromide (C₂H₅Br), also known as bromoethane, is an important organic compound. It can be prepared in three main ways:
- From ethanol by nucleophilic substitution (SN2 reaction)
- From ethane by free radical substitution
- From ethene by electrophilic addition reaction
Each method involves a different type of organic reaction such as substitution, addition, or free radical mechanism. Understanding these reactions helps students score better in board exams and competitive exams.
Additional Formulas:
Ethyl Bromide from Ethyl Alcohol, Ethane & Ethene related FAQs
1. How is ethyl bromide prepared in the laboratory?
It is prepared by reacting ethanol with hydrobromic acid (HBr).
2. What type of reaction is bromination of ethane?
It is a free radical substitution reaction.
3. Is ethyl bromide formed by addition or substitution?
It can be formed by both, depending on starting compound.
4. Why does ethanol follow SN2 reaction?
Because it is a primary alcohol.
5. What is the formula of ethyl bromide?
The formula is C₂H₅Br.